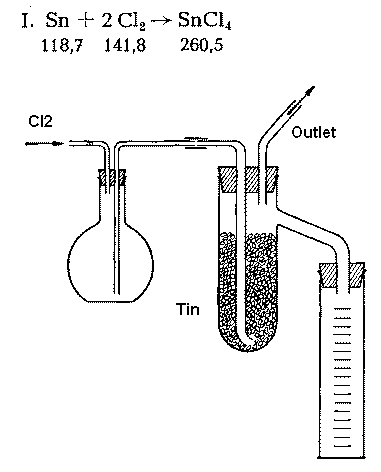
Sciencemadness Discussion Board - Small scale preparation of Stannic Chloride (SnCl4) - Powered by XMB 1.9.11
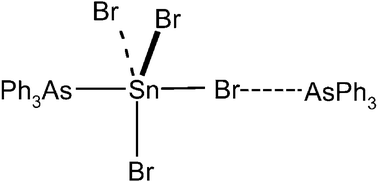
Tin(iv) halide complexes of AsPh3: The structures of trans-SnCl4(AsPh3)2 and SnBr4(AsPh3)·AsPh3 - Dalton Transactions (RSC Publishing)

SnCl4-organic base: Highly efficient catalyst system for coupling reaction of CO2 and epoxides - ScienceDirect

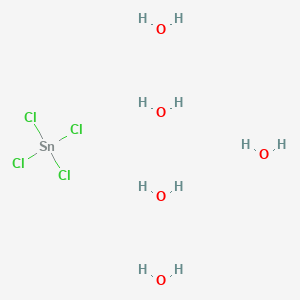
SOLVED: tin chloride dissolve in water according to sncl4->sn4+ 4cl-what is he boiling point of the solution when 0.2605 g of sncl4 molar mass 260.5 g/mol is dissolved in 10g of h20(
![Chemistry on Twitter: "Synthesis of functionalized tetrahydropyridines by SnCl4-mediated [4+2] cycloaddition between donor-acceptor cyclobutanes and nitriles. https://t.co/CXSWLKXbTY https://t.co/EKuMgRmicU" / Twitter Chemistry on Twitter: "Synthesis of functionalized tetrahydropyridines by SnCl4-mediated [4+2] cycloaddition between donor-acceptor cyclobutanes and nitriles. https://t.co/CXSWLKXbTY https://t.co/EKuMgRmicU" / Twitter](https://pbs.twimg.com/media/EIOjQHTW4AAHrLK.png)
Chemistry on Twitter: "Synthesis of functionalized tetrahydropyridines by SnCl4-mediated [4+2] cycloaddition between donor-acceptor cyclobutanes and nitriles. https://t.co/CXSWLKXbTY https://t.co/EKuMgRmicU" / Twitter

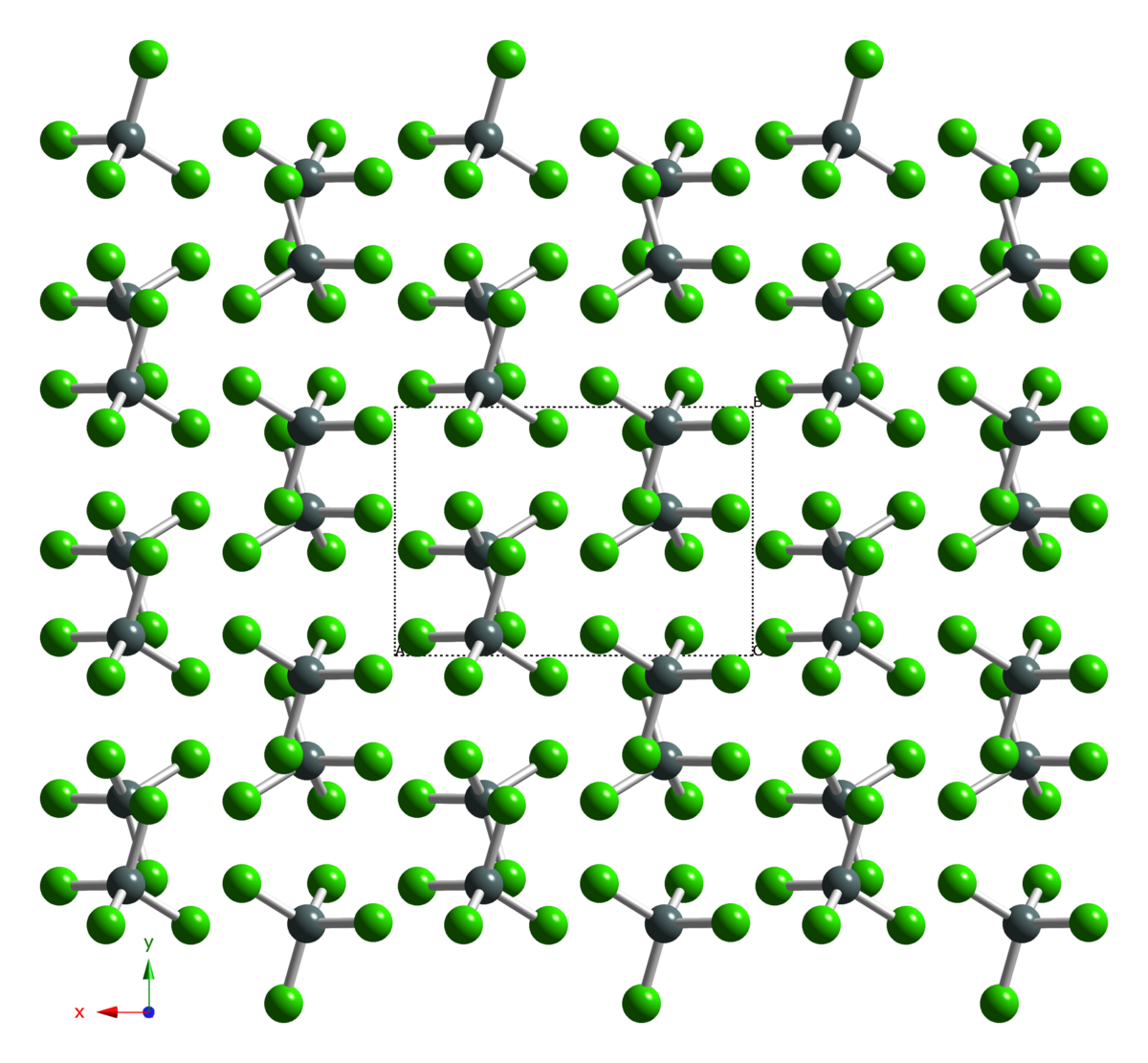



![PDF] Me3P complexes of p-block Lewis acids SnCl4, SnCl3+ and SnCl2(2+). | Semantic Scholar PDF] Me3P complexes of p-block Lewis acids SnCl4, SnCl3+ and SnCl2(2+). | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/edb67c01e87812d9fb9e93a78243b0ea63a687b8/2-Figure1-1.png)